Learning Outcomes:
i. Recall and define isomerism and its significance in organic chemistry.
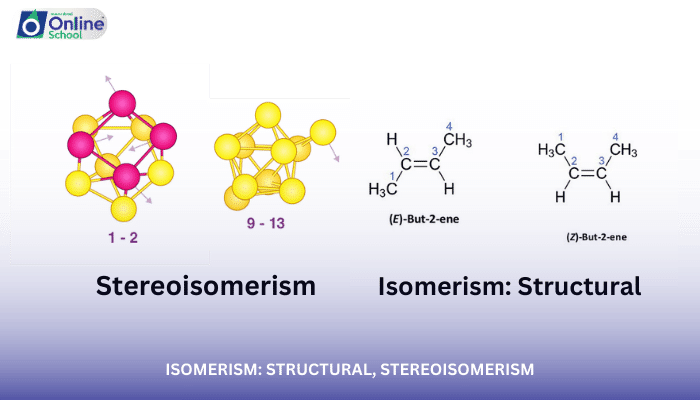
ii. Differentiate between structural isomerism and stereoisomerism based on their structural characteristics.
iii. Identify and classify structural isomers using examples such as butane and 2-methylpropane.
iv. Recognize and differentiate between enantiomers, diastereomers, and conformational isomers as types of stereoisomers.
v. Appreciate the importance of understanding isomerism for predicting the properties and reactivity of organic compounds.
Introduction
Isomerism, a fundamental concept in organic chemistry, refers to the existence of compounds with the same molecular formula but different structural arrangements. This lesson delves into the realm of isomerism, exploring the distinctions between structural isomerism and stereoisomerism.
i. Structural Isomerism: Different Structural Arrangements
Structural isomers, the simplest form of isomerism, have different arrangements of atoms within their molecules. They can be classified into two main categories:
Chain Isomerism: The carbon atoms form different types of chains, such as straight-chain and branched-chain isomers. For instance, butane (CH3CH2CH2CH3) and 2-methylpropane (CH3CH2CH(CH3)2) are chain isomers.
Positional Isomerism: The functional groups occupy different positions within the carbon chain. For example, propanol (CH3CH2CH2OH) and 2-propanol (CH3CH(OH)CH3) are positional isomers.
ii. Stereoisomerism: Different Spatial Arrangements
Stereoisomers, a more complex form of isomerism, have the same molecular formula and connectivity but different three-dimensional arrangements of their atoms. These isomers cannot be superimposed on each other.
Enantiomers: Enantiomers are non-superimposable mirror images of each other. They have identical physical and chemical properties except for their interaction with plane-polarized light. For instance, lactic acid (CH3CHOHCOOH) exists as two enantiomers, D-lactic acid and L-lactic acid.
Diastereomers: Diastereomers are non-superimposable stereoisomers that are not mirror images of each other. They have distinct physical and chemical properties. For example, 2,3-dichloropropane (CH3CHClCH2Cl) has two diastereomers.
Conformational Isomers: Conformational isomers arise from the rotation around single bonds in organic molecules. They represent different energy states of the same molecule and interconvert easily. For instance, ethane (CH3CH3) has two conformational isomers, staggered and eclipsed conformations.
iii. Significance of Understanding Isomerism
Understanding isomerism is crucial for predicting the properties and reactivity of organic compounds:
Physical Properties: Different isomers can exhibit distinct physical properties, such as melting point, boiling point, and density.
Chemical Reactivity: Isomers can react differently with different reagents due to their different spatial arrangements.
Biological Activity: Enantiomers of the same drug can have different biological activities, with one enantiomer being therapeutically active while the other may be inactive or even harmful.
Isomerism, with its diverse forms and implications, is a fundamental concept in organic chemistry. Understanding the different types of isomers, their structural distinctions, and their significance is essential for comprehending the properties, reactivity, and biological effects of organic compounds.